Properties of Water
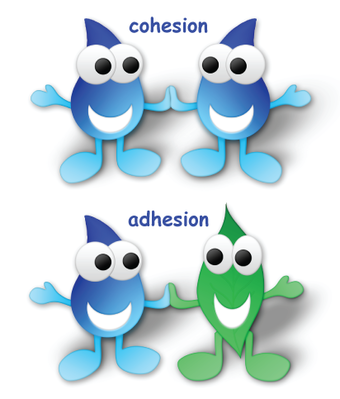
Cohesion and Surface Tension
Have you ever filled a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water forms a dome-like shape above the rim of the glass. This dome-like shape forms due to the water molecules’ cohesive properties, or their tendency to stick to one another.
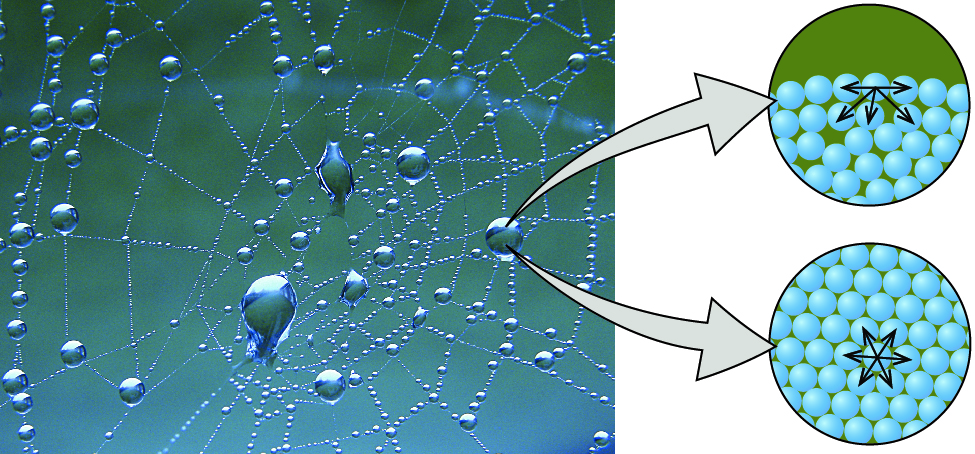
Water Drops
A water drop is composed of water molecules that like to stick together-an example of the property of cohesion. In the picture of a spider web above, the water droplets are stuck to the web-an example of the property of adhesion. Also noticeable in this picture is the effect that gravity has on the water drops. Gravity is working against both adhesion and cohesion, trying to pull the water drop downward. Adhesion and cohesion are winning the battle so far, as the drops are sticking to the web.


Hydrogen Bonds
Collectively, hydrogen bonds hold water molecules together, a phenomenon called cohesion
Think Magnets
Water is highly cohesive—it is the highest of the non-metallic liquids. Water is sticky and clumps together into drops because of its cohesive properties, but chemistry and electricity are involved at a more detailed level to make this possible. More precisely, the positive and negative charges of the hydrogen and oxygen atoms that make up water molecules makes them attracted to each other. If you’ve played with bar magnets you will know that the north pole of one magnet will repel the north pole of another magnet, but it will attract the south pole of another magnet. Opposite magnetic poles attract one another much like positively charged atoms attract negatively charged atoms in water molecules.
